Varlitinib Downregulates HER/ERK Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells
Abstract
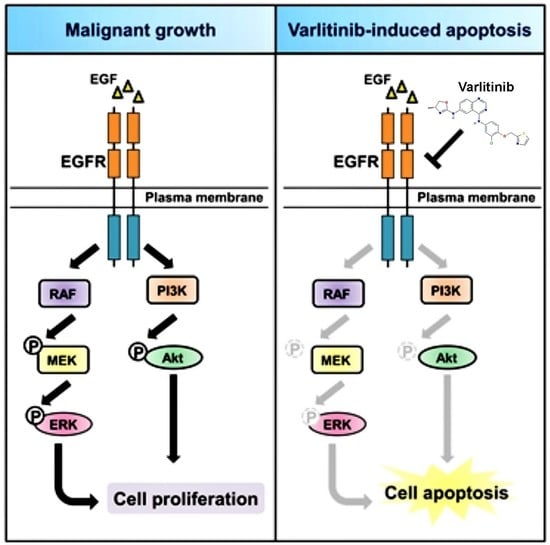
:1. Introduction
2. Results
2.1. Varlitinib Exerts Anti-Proliferation Ability and Induces Apoptosis
2.2. Varlitinib Suppresses MEK/ERK Pathway in TNBC Cells
2.3. Varlitinib Induces Apoptosis through ERK Inhibition in TNBC Cells
2.4. Varlitinib Inhibits Migration, Invasion and Mammosphere Formation of TNBC Cells
2.5. Varlitinib Shows Anti-Tumor Effect in TNBC Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Reagents and Transfection
4.2. Western Blot Analysis
4.3. MTT Assay
4.4. Flow Cytometry Analysis
4.5. Migration and Invasion Assays
4.6. Mammosphere Assay
4.7. Xenograft Tumor Growth
4.8. Immunohistochemical Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pareja, F.; Geyer, F.C.; Marchio, C.; Burke, K.A.; Weigelt, B.; Reis-Filho, J.S. Triple-negative breast cancer: The importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer 2016, 2, 16036. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, M.; Xie, J.; Wang, Z.; Wang, B.; Zhang, S.; Wang, L.; Cao, J.; Tao, Z.; Li, T.; et al. Chemotherapy of metastatic triple negative breast cancer: Experience of using platinum-based chemotherapy. Oncotarget 2015, 6, 43135–43143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwman, P.; Jonkers, J. Molecular pathways: How can BRCA-mutated tumors become resistant to PARP inhibitors? Clin. Cancer Res. 2014, 20, 540–547. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Jung, H.H.; Do, I.G.; Bae, S.; Lee, S.K.; Kim, S.W.; Lee, J.E.; Nam, S.J.; Ahn, J.S.; Park, Y.H.; et al. Prognostic value of ERBB4 expression in patients with triple negative breast cancer. BMC Cancer 2016, 16, 138. [Google Scholar] [CrossRef]
- Corkery, B.; Crown, J.; Clynes, M.; O’Donovan, N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann. Oncol. 2009, 20, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.Y.; La Choi, Y.; Kim, S.; Kim, M.; Kim, J.; Jung, S.P.; Choi, M.Y.; Lee, S.K.; Kil, W.H.; Lee, J.E.; et al. HER3 status by immunohistochemistry is correlated with poor prognosis in hormone receptor-negative breast cancer patients. Breast Cancer Res. Treat. 2013, 139, 741–750. [Google Scholar] [CrossRef]
- Tebbutt, N.; Pedersen, M.W.; Johns, T.G. Targeting the ERBB family in cancer: Couples therapy. Nat. Rev. Cancer 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Nam, H.J.; Kim, H.P.; Yoon, Y.K.; Song, S.H.; Min, A.R.; Han, S.W.; Im, S.A.; Kim, T.Y.; Oh, D.Y.; Bang, Y.J. The irreversible pan-HER inhibitor PF00299804 alone or combined with gemcitabine has an antitumor effect in biliary tract cancer cell lines. Investig. New Drugs 2012, 30, 2148–2160. [Google Scholar] [CrossRef]
- Adams, R.; Brown, E.; Brown, L.; Butler, R.; Falk, S.; Fisher, D.; Kaplan, R.; Quirke, P.; Richman, S.; Samuel, L.; et al. Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild-type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4-D): A phase 2-3 randomised trial. Lancet Gastroenterol. Hepatol. 2018, 3, 162–171. [Google Scholar] [CrossRef]
- Wang, X.; Batty, K.M.; Crowe, P.J.; Goldstein, D.; Yang, J.L. The Potential of panHER Inhibition in Cancer. Front. Oncol. 2015, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Feldinger, K.; Kong, A. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer 2015, 7, 147–162. [Google Scholar] [PubMed]
- Bennouna, J.; Moreno Vera, S.R. Afatinib-based combination regimens for the treatment of solid tumors: Rationale, emerging strategies and recent progress. Future Oncol. 2016, 12, 355–372. [Google Scholar] [CrossRef]
- Yang, J.C.; Sequist, L.V.; Zhou, C.; Schuler, M.; Geater, S.L.; Mok, T.; Hu, C.P.; Yamamoto, N.; Feng, J.; O’Byrne, K.; et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: Post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann. Oncol. 2016, 27, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Im, S.; Lee, K.; Kim, J.W.; Lee, K.; Han, S.; Kim, T.; Choi, I.S.; Oh, D.; Lee, N.; et al. 664p Phase IIa study to evaluate the biological activity of aslan001 in HER-1/2 co-expressing or her-2 amplified advanced gastric cancer. Ann. Oncol. 2014, 25, iv226. [Google Scholar] [CrossRef]
- Lee, S.C.; Chen, S.C.; Dai, M.S.; Lee, G.E.; Liu, C.L.; Chan, A.; Chang, H.K.; Tseng, L.M.; Chay, W.Y.; Chow, L.W.C.; et al. 102PMulticenter phase 2 trial of varlitinib versus lapatinib in combination with capecitabine in patients with HER2+ metastatic breast cancer (MBC) who failed prior trastuzumab therapy. Ann. Oncol. 2017, 28, mdx654.010. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar]
- Harding, J.; Burtness, B. Cetuximab: An epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today 2005, 41, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Mayer, E.L.; Esteva, F.J.; Ma, C.X.; Liu, M.C.; Storniolo, A.M.; Rimawi, M.F.; Forero-Torres, A.; et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J. Clin. Oncol. 2012, 30, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Ibrahim, M.F.K.; Fanning, K.; Cremona, M.; Morgan, C.; Hennessy, B.; Solca, F.; Crown, J.; O’Donovan, N. Biomarkers for afatinib and dasatinib treatment in triple negative breast cancer. Ann. Oncol. 2016, 27, 110P. [Google Scholar] [CrossRef]
- Mullooly, M.; Conklin, D.; McGowan, P.M.; O’Brien, N.A.; O’Donovan, N.; Slamon, D.J.; Crown, J.; Finn, R.S.; Duffy, M.J. Neratinib to inhibit the growth of triple-negative breast cancer cells. J. Clin. Oncol. 2015, 33, 1099. [Google Scholar]
- Schuler, M.; Awada, A.; Harter, P.; Canon, J.L.; Possinger, K.; Schmidt, M.; De Greve, J.; Neven, P.; Dirix, L.; Jonat, W.; et al. A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Res. Treat. 2012, 134, 1149–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikediobi, O.N.; Davies, H.; Bignell, G.; Edkins, S.; Stevens, C.; O’Meara, S.; Santarius, T.; Avis, T.; Barthorpe, S.; Brackenbury, L.; et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 2006, 5, 2606–2612. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Torres, N.M.; Tao, A.; Gao, Y.; Luo, L.; Li, Q.; de Stanchina, E.; Abdel-Wahab, O.; Solit, D.B.; Poulikakos, P.I.; et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine Their Sensitivity to Pharmacologic Inhibition. Cancer Cell 2015, 28, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Price, T.J.; Mohyieldin, O.; Borg, M.; Townsend, A.; Hardingham, J.E. KRAS G13D Mutation and Sensitivity to Cetuximab or Panitumumab in a Colorectal Cancer Cell Line Model. Gastrointest. Cancer Res. 2014, 7, 23–26. [Google Scholar] [PubMed]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov 2014, 13, 928–942. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Franklin, R.A.; Montalto, G.; Cervello, M.; Libra, M.; Candido, S.; Malaponte, G.; et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: How mutations can result in therapy resistance and how to overcome resistance. Oncotarget 2012, 3, 1068–1111. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, A.; Gallego, E.; de Luque, V.; Perez-Rivas, L.G.; Vicioso, L.; Ribelles, N.; Lozano, J.; Alba, E. Lack of evidence for KRAS oncogenic mutations in triple-negative breast cancer. BMC Cancer 2010, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Sheng, S.; Pardee, A.B. Metastasis and AKT activation. Cell Cycle 2008, 7, 2991–2996. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Zotano, A.; Mayer, I.A.; Arteaga, C.L. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016, 35, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol 2017, 14, 611. [Google Scholar] [CrossRef]
- Kim, S.B.; Dent, R.; Im, S.A.; Espie, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Van Sebille, Y.Z.A.; Gibson, R.J.; Wardill, H.R.; Bowen, J.M. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: Chloride secretion as a mechanistic hypothesis. Cancer Treat. Rev. 2015, 41, 646–652. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, T.T.; Chu, P.Y.; Huang, C.T.; Lee, C.H.; Wang, W.L.; Lau, K.Y.; Tsai, W.C.; Chao, T.I.; Su, J.C.; et al. The tyrosine kinase inhibitor nintedanib activates SHP-1 and induces apoptosis in triple-negative breast cancer cells. Exp. Mol. Med. 2017, 49, e366. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Chu, P.-Y.; Huang, C.-T.; Chen, J.-L.; Yang, H.-P.; Wang, W.-L.; Lau, K.-Y.; Lee, C.-H.; Lan, T.-Y.; Huang, T.-T.; et al. Varlitinib Downregulates HER/ERK Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells. Cancers 2019, 11, 105. https://doi.org/10.3390/cancers11010105
Liu C-Y, Chu P-Y, Huang C-T, Chen J-L, Yang H-P, Wang W-L, Lau K-Y, Lee C-H, Lan T-Y, Huang T-T, et al. Varlitinib Downregulates HER/ERK Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells. Cancers. 2019; 11(1):105. https://doi.org/10.3390/cancers11010105
Chicago/Turabian StyleLiu, Chun-Yu, Pei-Yi Chu, Chun-Teng Huang, Ji-Lin Chen, Hsiu-Ping Yang, Wan-Lun Wang, Ka-Yi Lau, Chia-Han Lee, Tien-Yun Lan, Tzu-Ting Huang, and et al. 2019. "Varlitinib Downregulates HER/ERK Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells" Cancers 11, no. 1: 105. https://doi.org/10.3390/cancers11010105