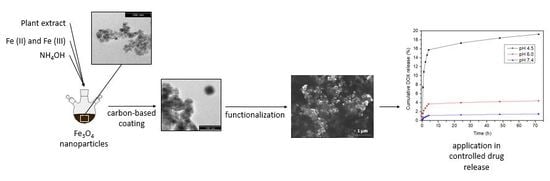
Carbon-Based Magnetic Nanocarrier for Controlled Drug Release: A Green Synthesis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Samples
2.2. Methods
2.2.1. Extracts Preparation
2.2.2. Magnetic Core Synthesis and Coating
2.2.3. Functionalization
2.2.4. Drug Loading and Release
3. Results and Discussion
3.1. Magnetic Core Synthesis and Coating
3.2. Functionalization
3.3. Drug Loading and Release
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teja, A.S.; Koh, P. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly Hydrophilic Luminescent Magnetic Mesoporous Carbon Nanospheres for Controlled Release of Anticancer Drug and Multimodal Imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; Román, J.S.; Goya, G.F.; Hernández, R.; Mijangos, C. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: From preparation to in vitro studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. A green and facile approach for synthesis of magnetite nanoparticles. J. Nanosci. Nanotechnol. 2012, 2, 208–213. [Google Scholar] [CrossRef]
- Shah, S.; Dasgupta, S.; Chakraborty, M.; Vadakkekara, R.; Hajoori, M. Green synthesis of iron nanoparticles using plant extracts. Int. J. Biol. Pharm. Res. 2014, 5, 549–552. [Google Scholar]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Bahiyah, N.; Khairudin, A.B.; Mohamad, S.E.B.; Lee, K.X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Harshiny, M.; Iswarya, C.N.; Matheswaran, M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. 2015, 286, 744–749. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Mallavarapu, M. Characterization of iron-polyphenol complex nanoparticles synthesized by Sage (Salvia officinalis) leaves. Environ. Technol. Innov. 2015, 4, 92–97. [Google Scholar] [CrossRef]
- Makarov, V.V.; Makarova, S.S.; Love, A.J.; Sinitsyna, O.V.; Dudnik, A.O.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants. Langmuir 2014, 30, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cabanas, M.; López-García, M.; Barriada, J.L.; Herrero, R.; Sastre de Vicente, M.E. Green synthesis of iron oxide nanoparticles. Development of magnetic hybrid materials for efficient As(V) removal. Chem. Eng. J. 2016, 301, 83–91. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, M.; Namvar, F.; Ahmad, M.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Gangadhara, S.; Venkateswarlu, P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity. Appl. Nanosci. 2016, 6, 797–802. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Al-kalifawi, E.J. Green synthesis of magnetite iron oxide nanoparticles by using Al-Abbas’s (A.S.) Hund Fruit (Citrus medica) var. Sarcodactylis Swingle extract and used in Al-’alqami river water treatment. J. Nat. Sci. Res. 2015, 5, 125–135. [Google Scholar]
- Liu, W.; Liu, Y.; Yan, X.; Yong, G.; Xu, Y.; Liu, S. One-pot synthesis of yolk-shell mesoporous carbon spheres with high magnetisation. J. Mater. Chem. A 2014, 2, 9600–9606. [Google Scholar] [CrossRef]
- Righetto, L. Doxorrubicina—Bula Doxorrubicina. 2013. Available online: https://www.4medic.com.br/bulario/D/doxorrubicina/ (accessed on 6 September 2018).
- Du, J.-Z.; Du, X.-J.; Mao, C.-Q.; Wang, J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus Monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in vitro antioxidant properties of edible wild greens in Iberian Peninsula traditional diet. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.S.; Rodrigues, R.O.; Silva, A.M.T.; Tavares, P.B.; Carvalho, A.M.C.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites towards catalytic wet peroxide oxidation of the liquid effluent from a mechanical biological treatment plant for municipal solid waste. Appl. Catal. B Environ. 2017, 219, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef]
- Das, C.; Bose, S. Advanced Ceramic Membranes and Applications; CRC Press: New York, NY, USA, 2017. [Google Scholar]
- Promega Buffers for Biochemical Reactions. Protoc. Appl. Guid. Available online: https://worldwide.promega.com/resources/product-guides-and-selectors/protocols-and-applications-guide/buffers-for-biochemical-reactions/ (accessed on 25 October 2018).
- Rodrigues, R.; Baldi, G.; Doumett, S.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.; Ferreira, I.; Lima, R.; Silva, A.; et al. A Tailor-made protocol to synthesize yolk-shell graphene-based magnetic nanoparticles for nanomedicine. J. Carbon Res. 2018, 4, 16. [Google Scholar] [CrossRef]
- Bagavathi, M.; Ramar, A.; Saraswathi, R. Fe3O4–carbon black nanocomposite as a highly efficient counter electrode material for dye-sensitized solar cell. Ceram. Int. 2016, 42, 13190–13198. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.-W.; Chen, J.; Yu, B.-X.; Hu, X.-Y. Carboxyl and negative charge-functionalized superparamagnetic nanochains with amorphous carbon shell and magnetic core: Synthesis and their application in removal of heavy metal ions. Nanoscale 2011, 3, 4600–4603. [Google Scholar] [CrossRef]
- Sezgin, Z.; Yüksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gyulai, G.; Magyar, A.; Rohonczy, J.; Orosz, J.; Yamasaki, M.; Bősze, S.; Kiss, É. Preparation and characterization of cationic Pluronic for surface modification and functionalization of polymeric drug delivery nanoparticles. Express Polym. Lett. 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Pradhan, L.; Srivastava, R.; Bahadur, D. pH- and thermosensitive thin lipid layer coated mesoporous magnetic nanoassemblies as a dual drug delivery system towards thermochemotherapy of cancer. Acta Biomater. 2014, 10, 2976–2987. [Google Scholar] [CrossRef] [PubMed]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.R.P.; Rodrigues, R.O.; Barros, L.; Ferreira, I.C.F.R.; Marchesi, L.F.; Koneracka, M.; Jurikova, A.; Zavisova, V.; Gomes, H.T. Carbon-Based Magnetic Nanocarrier for Controlled Drug Release: A Green Synthesis Approach. C 2019, 5, 1. https://doi.org/10.3390/c5010001
Oliveira JRP, Rodrigues RO, Barros L, Ferreira ICFR, Marchesi LF, Koneracka M, Jurikova A, Zavisova V, Gomes HT. Carbon-Based Magnetic Nanocarrier for Controlled Drug Release: A Green Synthesis Approach. C. 2019; 5(1):1. https://doi.org/10.3390/c5010001
Chicago/Turabian StyleOliveira, Jessica R. P., Raquel O. Rodrigues, Lillian Barros, Isabel C. F. R. Ferreira, Luís F. Marchesi, Martina Koneracka, Alena Jurikova, Vlasta Zavisova, and Helder T. Gomes. 2019. "Carbon-Based Magnetic Nanocarrier for Controlled Drug Release: A Green Synthesis Approach" C 5, no. 1: 1. https://doi.org/10.3390/c5010001