Fire and the Distribution and Uncertainty of Carbon Sequestered as Aboveground Tree Biomass in Yosemite and Sequoia & Kings Canyon National Parks
Abstract
:1. Introduction
1.1. Potential Errors Related to Allometry
- (1)
- Tree allometric equations themselves—tree level allometric equations are usually derived from a small number of trees (typically <25, but often <10 [19]), and the effects of model selection can greatly influence results [20]. The allometric equations often have considerable standard errors—usually 10% to 30% of the range of tree diameters used to develop them.
- (2)
- Site-specific tree morphology—trees sampled for allometric equations usually represent only one portion of the species range. When the equations are applied elsewhere, differences in site productivity, disturbance history, and climate could yield errors due to different morphologies between the sampled and target populations.
- (3)
- Lack of large-diameter tree representation—few allometric equations are derived from samples of large trees, with very few tree dissections having occurred for trees >100 cm diameter at breast height (dbh). Therefore, the biomass of large-diameter trees must be estimated from those few proxy species that have been sampled at large diameters. The problem of accurately calculating the biomass of large-diameter trees is magnified by their more complex and variable crown architecture [21,22]. Importantly, large-diameter trees contain a large proportion of the biomass of Sierra Nevada forests [11]. Therefore, in areas where large-diameter trees exist, particularly where individuals reach maximum sizes much greater than any previously dissected, biomass estimates could potentially have large uncertainties.
1.2. Potential Errors Related to Landscape Classification and Mapping
- (4)
- Landscape heterogeneity—landscape level estimates of carbon must be based on a sufficient number of plots to capture the range in vegetation conditions and their representation on the landscape [23]. Although the present study used a sufficient number of forest plots (1646), studies with too few plots to represent the landscape heterogeneity also may have errors related to the time since disturbance, a principal contributor to heterogeneity [24].
- (5)
- Vegetation type mismatch—when plot level data are scaled to the landscape, errors in vegetation cover assignment can yield errors in scaled biomass.
2. Materials and Methods
2.1. Study Area
2.2. Plot Data
2.3. Vegetation Mapping
2.4. Allometric Equations
2.5. Landscape Mapping
2.6. Density and Total Carbon
2.7. Fire History and Carbon Density
2.8. Scenarios
2.9. Comparison
3. Results
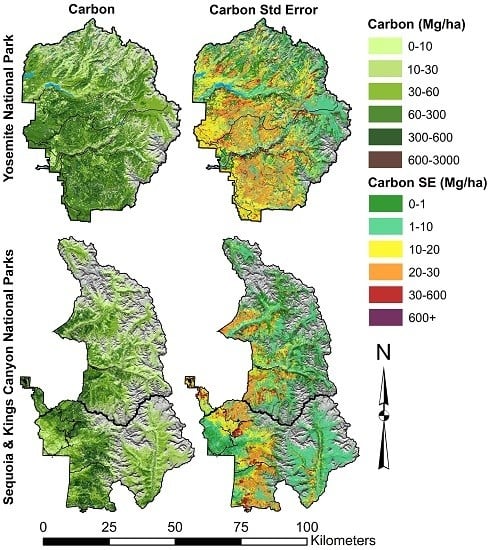
3.1. Aboveground Tree Carbon Stocks and Carbon Densities
3.2. Carbon Uncertainties
3.3. Fire History and Tree Carbon Density
3.4. Carbon Stability and Future Fire
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Taxa | Min. DBH (cm) | Max. DBH (cm) | Ceiling DBH (cm) | Component | Equation | a | b | SEE | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Abies concolor | 0 | 6.9999 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Abies concolor | 7 | 98 | 1000 | tree | Abies concolor | −2.5521 | 2.5043 | 0.16805 | [48] |
| Abies concolor | 98.0001 | 1000 | 1000 | bole | Abies procera | −3.0319 | 2.5812 | 0.1841 | [47] |
| Abies concolor | 98.0001 | 1000 | 111 | branch live | Abies pooled | −4.9318 | 2.5585 | 0.454 | [87] |
| Abies concolor | 98.0001 | 1000 | 111 | foliage | Abies pooled | −3.5458 | 1.9278 | 0.399 | [87] |
| Abies | 0 | 27.5 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Abies | 27.5001 | 100 | 1000 | tree | Abies magnifica | −4.3136 | 2.9121 | 0.22074 | [48] |
| Abies | 100.0001 | 1000 | 1000 | bole | Abies procera | −3.0319 | 2.5812 | 0.1841 | [47] |
| Abies | 100.0001 | 1000 | 111 | branch live | Abies pooled | −4.9318 | 2.5585 | 0.454 | [87] |
| Abies | 100.0001 | 1000 | 111 | foliage | Abies pooled | −3.5458 | 1.9278 | 0.399 | [87] |
| Abies magnifica | 0 | 27.5 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Abies magnifica | 27.5001 | 100 | 1000 | tree | Abies magnifica | −4.3136 | 2.9121 | 0.22074 | [48] |
| Abies magnifica | 100.0001 | 1000 | 1000 | bole | Abies procera | −3.0319 | 2.5812 | 0.1841 | [47] |
| Abies magnifica | 100.0001 | 1000 | 111 | branch live | Abies pooled | −4.9318 | 2.5585 | 0.454 | [87] |
| Abies magnifica | 100.0001 | 1000 | 111 | foliage | Abies pooled | −3.5458 | 1.9278 | 0.399 | [87] |
| Acer macrophyllum | 0 | 7.5999 | 1000 | tree | soft maple/birch | −2.0332 | 2.3651 | 0.491685 | [49] |
| Acer macrophyllum | 7.6 | 1000 | 1000 | bole bark | Acer macrophyllum | −4.5757 | 2.574 | 0.058 | [88] |
| Acer macrophyllum | 7.6 | 1000 | 1000 | bole wood | Acer macrophyllum | −3.4931 | 2.723 | 0.014 | [88] |
| Acer macrophyllum | 7.6 | 1000 | 1000 | branch dead | Acer macrophyllum | −3.8495 | 1.092 | 1.862 | [88] |
| Acer macrophyllum | 7.6 | 1000 | 1000 | branch live | Acer macrophyllum | −4.2613 | 2.43 | 0.225 | [88] |
| Acer macrophyllum | 7.6 | 1000 | 1000 | foliage | Acer macrophyllum | −3.7701 | 1.617 | 0.101 | [88] |
| Aesculus californica | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Alnus rhombifolia | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Arctostaphylos viscida | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Arctostaphylos viscida ssp. viscida | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Betula occidentalis | 0 | 1000 | 1000 | tree | soft maple/birch | −2.0332 | 2.3651 | 0.491685 | [49] |
| Calocedrus decurrens | 0 | 1000 | 1000 | tree | cedar/larch | −2.077 | 2.2592 | 0.294574 | [49] |
| Cercocarpus betuloides | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Cercocarpus ledifolius | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Cercis occidentalis | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Corylus cornuta var. californica | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Cornus nuttallii | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Fraxinus dipetala | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Fraxinus latifolia | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Fraxinus velutina | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Juniperus occidentalis | 0 | 1000 | 1000 | tree | Juniperus occidentalis | −5.6604 | 2.2462 | 0.1433 | [87] |
| Juniperus occidentalis var. australis | 0 | 1000 | 1000 | tree | Juniperus occidentalis | −5.6604 | 2.2462 | 0.1433 | [87] |
| Juniperus osteosperma | 0 | 1000 | 1000 | tree | Juniperus occidentalis | −5.6604 | 2.2462 | 0.1433 | [87] |
| Malus | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Pinus albicaulis | 0 | 10 | 1000 | tree | Pinus albicaulis | −0.389 | 1.1585 | 0.4045 | [89] |
| Pinus albicaulis | 10.0001 | 20 | 1000 | bole | Juniperus occidentalis | −8.3826 | 2.6378 | 0.159 | [87] |
| Pinus albicaulis | 10.0001 | 20 | 1000 | canopy | Pinus albicaulis | −1.3017 | 1.2991 | 0.483 | [89] |
| Pinus albicaulis | 20.0001 | 1000 | 1000 | tree | Juniperus occidentalis | −5.6604 | 2.2462 | 0.1433 | [87] |
| Pinus attenuata | 0 | 1000 | 1000 | tree | pine | −2.5678 | 2.4349 | 0.253781 | [49] |
| Pinus balfouriana ssp. austrina | 0 | 10 | 1000 | tree | Pinus albicaulis | −0.389 | 1.1585 | 0.4045 | [89] |
| Pinus balfouriana ssp. austrina | 10.0001 | 20 | 1000 | bole | Juniperus occidentalis | −8.3826 | 2.6378 | 0.159 | [87] |
| Pinus balfouriana ssp. austrina | 10.0001 | 20 | 1000 | canopy | Pinus albicaulis | −1.3017 | 1.2991 | 0.483 | [89] |
| Pinus balfouriana ssp. austrina | 20.0001 | 1000 | 1000 | tree | Juniperus occidentalis | −5.6604 | 2.2462 | 0.1433 | [87] |
| Pinus contorta var. murrayana | 0 | 19.9999 | 1000 | tree | Pinus contorta | −2.095 | 2.3909 | 0.4786 | [90] |
| Pinus contorta var. murrayana | 20 | 1000 | 1000 | tree | Pinus contorta | −1.0386 | 1.9294 | 0.3205 | [91] |
| Pinus jeffreyi | 0 | 22.3999 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Pinus jeffreyi | 22.4 | 133.1 | 1000 | bole | Pinus jeffreyi | −5.1108 | 2.952 | 0.204834 | [47] |
| Pinus jeffreyi | 22.4 | 1000 | 162 | branches dead | Pseudotsuga menziesii | −3.794 | 1.7503 | 0.728 | [87] |
| Pinus jeffreyi | 22.4 | 1000 | 162 | branches live | Pseudotsuga menziesii | −3.8938 | 2.1382 | 0.632 | [87] |
| Pinus jeffreyi | 22.4 | 1000 | 162 | foliage | Pseudotsuga menziesii | −3.0877 | 1.7009 | 0.695 | [87] |
| Pinus jeffreyi | 133.1001 | 1000 | 1000 | bole | Pseudotsuga menziesii | −2.2765 | 2.4247 | 0.2415 | [47] |
| Pinus lambertiana | 0 | 8.6999 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Pinus lambertiana | 8.7 | 179.6 | 1000 | bole | Pinus lambertiana | −3.6973 | 2.6863 | 0.193513 | [47] |
| Pinus lambertiana | 8.7 | 1000 | 162 | branches dead | Pseudotsuga menziesii | −3.794 | 1.7503 | 0.728 | [87] |
| Pinus lambertiana | 8.7 | 1000 | 162 | branches live | Pseudotsuga menziesii | −3.8938 | 2.1382 | 0.632 | [87] |
| Pinus lambertiana | 8.7 | 1000 | 162 | foliage | Pseudotsuga menziesii | −3.0877 | 1.7009 | 0.695 | [87] |
| Pinus lambertiana | 179.6001 | 1000 | 1000 | bole | Pseudotsuga menziesii | −2.2765 | 2.4247 | 0.2415 | [47] |
| Pinus monophylla | 0 | 1000 | 1000 | tree | pine | −2.5678 | 2.4349 | 0.253781 | [49] |
| Pinus monticola | 0 | 19.9999 | 1000 | tree | Pinus contorta | −2.095 | 2.3909 | 0.4786 | [90] |
| Pinus monticola | 20 | 1000 | 1000 | tree | Pinus contorta | −1.0386 | 1.9294 | 0.3205 | [91] |
| Pinus | 0 | 1000 | 1000 | tree | pine | −2.5678 | 2.4349 | 0.253781 | [49] |
| Pinus ponderosa | 0 | 15.4999 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Pinus ponderosa | 15.5 | 79.5 | 1000 | tree | Pinus ponderosa | −3.2673 | 2.582 | 0.1266 | [87] |
| Pinus ponderosa | 79.5001 | 1000 | 1000 | bole | Pseudotsuga menziesii | −2.2765 | 2.4247 | 0.2415 | [47] |
| Pinus ponderosa | 79.5001 | 1000 | 162 | branches dead | Pseudotsuga menziesii | −3.794 | 1.7503 | 0.728 | [87] |
| Pinus ponderosa | 79.5001 | 1000 | 162 | branches live | Pseudotsuga menziesii | −3.8938 | 2.1382 | 0.632 | [87] |
| Pinus ponderosa | 79.5001 | 1000 | 162 | foliage | Pseudotsuga menziesii | −3.0877 | 1.7009 | 0.695 | [87] |
| Pinus sabiniana | 0 | 1000 | 1000 | tree | pine | −2.5678 | 2.4349 | 0.253781 | [49] |
| Platanus racemosa | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Populus balsamifera | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Populus balsamifera ssp. trichocarpa | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Populus tremuloides | 0 | 36 | 1000 | tree | Populus tremuloides | −2.1461 | 2.242 | 0.3205 | [92] |
| Populus tremuloides | 36.0001 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Prunus emarginata | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Prunus virginiana var. demissa | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Pseudotsuga menziesii | 0 | 1000 | 1000 | tree | Pseudotsuga menziesii | −2.2543 | 2.4435 | 0.218712 | [49] |
| Quercus chrysolepis | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus douglasii | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus kelloggii | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus lobata | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus x moreha | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus wislizeni | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Quercus wislizeni var. wislizeni | 0 | 1000 | 1000 | tree | hard maple/oak/hickory/beech | −2.0407 | 2.4342 | 0.236483 | [49] |
| Rhamnus californica | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Rhamnus ilicifolia | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| Salix laevigata | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix lasiolepis | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix lucida | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix lucida ssp. lasiandra | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix melanopsis | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Salix scouleriana | 0 | 1000 | 1000 | tree | aspen/alder/cottonwood/willow | −2.3381 | 2.3867 | 0.507441 | [49] |
| Sequoiadendron giganteum | 0 | 96.7999 | 1000 | tree | cedar/larch | −2.077 | 2.2592 | 0.294574 | [49] |
| Sequoiadendron giganteum | 96.8 | 1000 | 1000 | bole | Sequoiadendron giganteum | −2.8134 | 2.4019 | 0.254442 | [47] |
| Torreya californica | 0 | 1000 | 1000 | tree | mixed hardwood | −2.545 | 2.4835 | 0.360458 | [49] |
| generic tree species | 0 | 1000 | 1000 | tree | pine | −2.5678 | 2.4349 | 0.253781 | [49] |
| Tsuga mertensiana | 0 | 11.4999 | 1000 | tree | small conifer | −1.8516 | 2.3701 | 0.1191 | [86] |
| Tsuga mertensiana | 11.5 | 1000 | 1000 | bole | Tsuga mertensiana | −3.2801 | 2.5915 | 0.195028 | [47] |
| Tsuga mertensiana | 11.5 | 1000 | 1000 | branch live | Tsuga mertensiana | −5.2655 | 2.6045 | 0.122 | [87] |
| Tsuga mertensiana | 11.5 | 1000 | 1000 | branches dead | Tsuga mertensiana | −9.951 | 3.2845 | 0.11 | [87] |
| Tsuga mertensiana | 11.5 | 1000 | 1000 | foliage | Tsuga mertensiana | −3.8294 | 1.9756 | 0.158 | [87] |
| Umbellularia californica | 0 | 1000 | 1000 | tree | Umbellularia californica | −2.1313 | 2.3996 | 0.2497 | [93] |
References
- Hurteau, M.D.; Brooks, M.L. Short- and long-term effects of fire on carbon in US dry temperate forest ecosystems. BioScience 2011, 61, 139–146. [Google Scholar] [CrossRef]
- Swann, A.L.S.; Fung, I.Y.; Chiang, J.C.H. Mid-latitude afforestation shifts general circulation and tropical precipitation. Proc. Natl. Acad. Sci. USA 2012, 109, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.S.; Kolden, C.A.; Paveglio, T.B.; Cochrane, M.A.; Bowman, D.; Moritz, M.A.; Kliskey, A.D.; Alessa, L.; Hudak, A.T.; Hoffman, C.M.; et al. The science of firescapes: Achieving fire resilient communities. BioScience 2016, 66, 130–146. [Google Scholar] [CrossRef]
- Becker, K.M.L.; Lutz, J.A. Can low-severity fire reverse overstory compositional change in montane forests of the Sierra Nevada, USA? Ecosphere 2016, 7, e01484. [Google Scholar] [CrossRef]
- Chisholm, R.A.; Muller-Landau, H.C.; Abdul Rahman, K.; Bebber, D.P.; Bin, Y.; Bohlman, S.A.; Bourg, N.A.; Brinks, J.; Brokaw, N.; Bunyavejchewin, S.; et al. Scale-dependent relationships between species richness and ecosystem function in forests. J. Ecol. 2013, 101, 1214–1224. [Google Scholar] [CrossRef]
- Larson, A.J.; Lutz, J.A.; Gersonde, R.F.; Franklin, J.F.; Hietpas, F.F. Productivity influences the rate of forest structural development. Ecol. Appl. 2008, 18, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.D.; Battles, J.J.; Richardson, A.D.; Blodgett, C.A.; Wood, D.M.; Rastetter, E.B. Estimating uncertainty in ecosystem budget calculations. Ecosystems 2010, 13, 239–248. [Google Scholar] [CrossRef]
- Yanai, R.D.; Levine, C.R.; Green, M.B.; Campbell, J.L. Quantifying uncertainty in forest nutrient budgets. J. For. 2012, 110, 448–456. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Aguilar, S.; Hernandez, A.; Lao, S.; Perez, R. Error propagation and scaling for tropical biomass estimates. Philos. Trans. R. Soc. Lond. B 2004, 359, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Fasth, B.; Halpern, C.B.; Lutz, J.A. Uncertainty analysis: An evaluation metric for synthesis science. Ecosphere 2015, 6. [Google Scholar] [CrossRef]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 2012, 7, e36131. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A.; Schwindt, K.A.; Furniss, T.J.; Freund, J.A.; Swanson, M.E.; Hogan, K.I.; Kenagy, G.E.; Larson, A.J. Community composition and allometry of Leucothoe davisiae, Cornus sericea, and Chrysolepis sempervirens. Can. J. For. Res. 2014, 44, 677–683. [Google Scholar] [CrossRef]
- Gabrielson, A.T.; Larson, A.J.; Lutz, J.A.; Reardon, J.J. Biomass and burning characteristics of sugar pine cones. Fire Ecol. 2012, 8, 58–70. [Google Scholar] [CrossRef]
- Larson, A.J.; Cansler, C.A.; Cowdery, S.G.; Hiebert, S.; Furniss, T.J.; Swanson, M.E.; Lutz, J.A. Post-fire morel (Morchella) mushroom production, spatial structure, and harvest sustainability. For. Ecol. Manag. 2016, 377, 16–25. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Houghton, R.A.; Dos Santos Alvala, R.C.; Soares, J.V.; Yu, Y. Distribution of aboveground live biomass in the Amazon basin. Glob. Chang. Biol. 2007, 13, 816–837. [Google Scholar] [CrossRef]
- Erickson, H.E.; Soto, P.; Johnson, D.W.; Roath, B.; Hunsaker, C. Effects of vegetation patches on soil nutrient pools and fluxes within a mixed-conifer forest. For. Sci. 2005, 51, 211–220. [Google Scholar]
- Potter, C. The carbon budget of California. Environ. Sci. Policy 2010, 13, 373–383. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. Comprehensive Database of Diameter-Based Biomass Regressions for North American Tree Species; USDA Forest Service General Technical Report 2004, NE-319; USDA Forest Service: Newtown Square, PA, USA, 2003. [Google Scholar]
- Melson, S.L.; Harmon, M.E.; Fried, J.S.; Domingo, J.B. Estimates of live-tree carbon stores in the Pacific Northwest are sensitive to model selection. Carbon Balance Manag. 2011, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Sillett, S.C.; van Pelt, R. Trunk reiteration promotes epiphytes and water storage in an old-growth redwood forest canopy. Ecol. Monogr. 2007, 77, 335–359. [Google Scholar] [CrossRef]
- Van Pelt, R.; Sillett, S.C. Crown development throughout the lifespan of coastal Pseudotsuga menziesii, including a conceptual model for tall conifers. Ecol. Monogr. 2008, 78, 283–311. [Google Scholar] [CrossRef]
- Wilson, B.T.; Woodall, C.W.; Griffith, D.M. Imputing forest carbon stock estimates from inventory plots to a nationally continuous coverage. Carbon Balance Manag. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.D.; Kolden, C.A. Development of a historical multi-year land cover classification incorporating wildfire effects. Land 2014, 3, 1214–1231. [Google Scholar] [CrossRef]
- Kane, V.R.; Gersonde, R.F.; Lutz, J.A.; McGaughey, R.J.; Bakker, J.D.; Franklin, J.F. Patch dynamics and the development of structural and spatial heterogeneity in Pacific Northwest forests. Can. J. For. Res. 2011, 41, 2276–2291. [Google Scholar] [CrossRef]
- Kane, V.R.; Lutz, J.A.; Roberts, S.L.; Smith, D.F.; McGaughey, R.J.; Povak, N.A.; Brooks, M.L. Landscape-scale effects of fire severity on mixed-conifer and red fir forest structure in Yosemite National Park. For. Ecol. Manag. 2013, 287, 17–31. [Google Scholar] [CrossRef]
- Kane, V.R.; North, M.; Lutz, J.A.; Churchill, D.; Roberts, S.L.; Smith, D.F.; McGaughey, R.J.; Kane, J.T.; Brooks, M.L. Assessing fire-mediated change to forest spatial structure using a fusion of Landsat and airborne LiDAR data in Yosemite National Park. Remote Sens. Environ. 2014, 151, 89–101. [Google Scholar] [CrossRef]
- Lutz, J.A.; van Wagtendonk, J.W.; Franklin, J.F. Climatic water deficit, tree species ranges, and climate change in Yosemite National Park. J. Biogeogr. 2010, 37, 936–950. [Google Scholar] [CrossRef]
- Lutz, J.A.; Halpern, C.B. Tree mortality during early forest development: a long-term study of rates, causes, and consequences. Ecol. Monogr. 2006, 76, 257–275. [Google Scholar] [CrossRef]
- North, M.J.; Innes, J.; Zald, H. Comparison of thinning and prescribed fire restoration treatments to Sierran mixed-conifer historic conditions. Can. J. For. Res. 2007, 37, 331–342. [Google Scholar] [CrossRef]
- Van Wagtendonk, J.W.; Lutz, J.A. Fire regime attributes of wildland fires in Yosemite National Park, USA. Fire Ecol. 2007, 3, 34–52. [Google Scholar] [CrossRef]
- Lutz, J.A.; Key, C.H.; Kolden, C.A.; Kane, J.T.; van Wagtendonk, J.W. Fire frequency, area burned, and severity: A quantitative approach to defining a normal fire year. Fire Ecol. 2011, 7, 51–65. [Google Scholar] [CrossRef]
- Hurteau, M.D.; North, M.P. Fuel treatment effects on tree-based forest carbon storage and emissions under modeled wildfire scenarios. Front. Ecol. Environ. 2009, 7, 409–414. [Google Scholar] [CrossRef]
- Halpern, C.B.; Lutz, J.A. Canopy closure exerts weak controls on understory dynamics: A 30-year study of overstory-understory interactions. Ecol. Monogr. 2013, 83, 19–35. [Google Scholar] [CrossRef]
- Van Wagtendonk, J.W.; Fites-Kaufman, J. Sierra Nevada bioregion. In Fire in California’s Ecosystems; Sugihara, N.G., van Wagtendonk, J.W., Shaffer, K.E., Fites-Kaufman, J., Thode, A.E., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 264–294. [Google Scholar]
- Fites-Kaufman, J.; Rundel, P.; Stephenson, N.; Weixelman, D.A. Montane and subalpine vegetation of the Sierra Nevada and Cascade ranges. In Terrestrial Vegetation of California; Barbour, M.G., Keeler-Wolf, T., Schoenherr, A.A., Eds.; University of California Press: Berkeley, CA, USA, 2007; pp. 456–501. [Google Scholar]
- Kane, V.R.; Lutz, J.A.; Cansler, C.A.; Povak, N.A.; Churchill, D.; Smith, D.F.; Kane, J.T.; North, M.P. Water balance and topography predict fire and forest structure patterns. For. Ecol. Manag. 2015, 338, 1–13. [Google Scholar] [CrossRef]
- Scholl, A.E.; Taylor, A.H. Fire regimes, forest change, and self-organization in an old-growth mixed-conifer forest, Yosemite National Park, USA. Ecol. Appl. 2010, 20, 362–380. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.A.F.; Larson, A.J.; Lutz, J.A. Use of a forest reconstruction model to assess changes to Sierra Nevada mixed-conifer forest during the fire suppression era. For. Ecol. Manag. 2015, 354, 104–118. [Google Scholar] [CrossRef]
- Levine, C.R.; Cogbill, C.V.; Collins, B.M.; Larson, A.J.; Lutz, J.A.; North, M.P.; Restaino, C.M.; Safford, H.D.; Stephens, S.L.; Battles, J.J. Evaluating a new method for reconstructing forest conditions from General Land Office survey records. Ecol. Appl. 2017, in press. [Google Scholar]
- Matchett, J.R.; Lutz, J.A.; Tarnay, L.W.; Smith, D.G.; Becker, K.M.L.; Brooks, M.L. Impacts of Fire Management on Aboveground Tree Carbon Stocks in Yosemite and Sequoia & Kings Canyon National Parks; Natural Resources Report, NPS/SIEN/NRR-2015/910; National Park Service: Fort Collins, CO, USA, 2015. [Google Scholar]
- Keeler-Wolf, T.; Moore, P.E.; Reyes, E.T.; Menke, J.M.; Johnson, D.N.; Karavidas, D.L. Yosemite National Park Vegetation Classification and Mapping Project Report; Natural Resource Technical Report, NPS/YOSE/NRTR—2012/598; National Park Service: Fort Collins, CO, USA, 2012. [Google Scholar]
- Aerial Information Systems, Inc. USGS-NPS Vegetation Mapping Program, Sequoia & Kings Canyon National Parks Photo Interpretation Report; Aerial Information Systems, Inc.: Redlands, CA, USA, 2007. [Google Scholar]
- Stephens, S.L.; Lydersen, J.M.; Collins, B.M.; Fry, D.L.; Meyer, M.D. Historical and current landscape-scale ponderosa pine and mixed conifer forest structure in the southern Sierra Nevada. Ecosphere 2015, 6, 1–63. [Google Scholar] [CrossRef]
- Van de Water, K.M.; Safford, H.D. A summary of fire frequency estimates for California vegetation before Euro-American settlement. Fire Ecol. 2011, 7, 26–58. [Google Scholar] [CrossRef]
- Van Wagtendonk, J.W.; Moore, P.E. Fuel deposition rates of montane and subalpine conifers in the central Sierra Nevada, California, USA. For. Ecol. Manag. 2010, 259, 2122–2132. [Google Scholar] [CrossRef]
- Means, J.E.; Hansen, H.A.; Koerper, G.J.; Alaback, P.B.; Klopsch, M.W. Software for Computing Plant Biomass—Biopak Users Guide; USDA Forest Service General Technical Report 1994, PNW-GTR-340; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1994.
- Westman, W.E. Aboveground biomass, surface area, and production relations of red fir (Abies magnifica) and white fir (A. concolor). Can. J. For. Res. 1998, 17, 311–319. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Chojnacky, D.C.; Heath, L.S.; Birdsey, R.A. National-scale biomass estimators for United States tree species. For. Sci. 2003, 49, 12–35. [Google Scholar]
- Miller, L.M.; Meeuwig, R.O.; Budy, J.D. Biomass of Singleleaf Pinyon and Utah Juniper; USDA Forest Service, Intermountain Forest and Range Experimental Station Research Paper 1981, INT-273; Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1981.
- Van Pelt, R. Forest Giants of the Pacific Coast; University of Washington Press: Seattle, WA, USA, 2001. [Google Scholar]
- Demaerschaulk, J.P.; Omule, S.A.Y. Estimating breast height diameters from stump measurements in British Columbia. For. Chron. 1982, 58, 143–145. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Gonzalez, P.; Asner, G.P.; Battles, J.J.; Lefsky, M.A.; Waring, K.M.; Palace, M. Forest carbon densities and uncertainties from LiDAR, QuickBird, and field measurements in California. Remote Sens. Environ. 2010, 114, 1561–1575. [Google Scholar] [CrossRef]
- Baskerville, G.L. Use of logarithmic regression in the estimation of plant biomass. Can. J. For. Res. 1972, 2, 49–53. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.0; R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: http://www.R-project.org/ (accessed on 5 January 2013).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- PostGIS Development Team. PostGIS, Version 2.1; 2013. Available online: http://postgis.net (accessed on 5 January 2013).
- QGIS Development Team. QGIS, Version 2.4; 2013. Available online: http://qgis.net (accessed on 5 January 2013).
- Kane, V.R.; Cansler, C.A.; Povak, N.A.; Kane, J.T.; McGaughey, R.J.; Lutz, J.A.; Churchill, D.J.; North, M.P. Mixed severity fire effects within the Rim fire: Relative importance of local climate, fire weather, topography, and forest structure. For. Ecol. Manag. 2015, 358, 62–79. [Google Scholar] [CrossRef]
- Stavros, E.N.; Tane, Z.; Kane, V.R.; Veraverbeke, S.; McGaughey, R.J.; Lutz, J.A.; Ramirez, C.; Schimel, D. Unprecedented remote sensing data over the King and Rim megafires in the Sierra Nevada mountains of California. Ecology 2016, 97, 3244. [Google Scholar] [CrossRef] [PubMed]
- Safford, H.D.; van de Water, K.M. Using Fire Return Interval Departure (FRID) Analysis to Map Spatial and Temporal Changes in Fire Frequency on National Forest Lands in California; USDA Forest Service Research Paper 2014, PSW-RP-266; United States Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2014.
- Hann, W.J.; Strohm, D.J. Fire regime condition class and associated data for fire and fuels planning: methods and applications. In Fire, Fuel Treatments, and Ecological Restoration; Omi, P.N., Joyce, L.A., Eds.; USDA Forest Service Rocky Mountain Research Station: Fort Collins, CO, USA, 2003; pp. 397–434. [Google Scholar]
- Safford, H.D.; van de Water, K.M.; Schmidt, D. California Fire Return Interval Departure (FRID) Map, 2010 Version; USDA Forest Service, Pacific Southwest Region, and The Nature Conservancy: Albany, CA, USA, 2011. [Google Scholar]
- Van Wagtendonk, J.W.; van Wagtendonk, K.A.; Meyer, J.B.; Painter, K.J. The use of geographic information for fire management planning in Yosemite National Park. Appl. Geogr. 2002, 19, 19–39. [Google Scholar]
- Lutz, J.A.; Larson, A.J.; Freund, J.A.; Swanson, M.E.; Bible, K.J. The ecological importance of large-diameter trees to forest structural heterogeneity. PLoS ONE 2013, 8, e82784. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Collins, B.M.; Lutz, J.A.; Stephens, S.L.; van Wagtendonk, J.W.; Yasuda, D.A. Differences in wildfires among ecoregions and land management agencies in the Sierra Nevada region, California, USA. Ecosphere 2012, 3, 80. [Google Scholar] [CrossRef]
- Lutz, J.A. The evolution of long-term data for forestry: Large temperate research plots in an era of global change. Northwest Sci. 2015, 89, 255–269. [Google Scholar] [CrossRef]
- Lutz, J.A.; Larson, A.J.; Furniss, T.J.; Freund, J.A.; Swanson, M.E.; Donato, D.C.; Bible, K.J.; Chen, J.; Franklin, J.F. Spatially non-random tree mortality and ingrowth maintain equilibrium pattern in an old-growth Pseudotsuga-Tsuga forest. Ecology 2014, 95, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, T.W.; Shook, C.D.; Keeley, J.E. Estimating aboveground biomass for broadleaf woody plants and young conifers in Sierra Nevada, California, forests. West. J. Appl. For. 2010, 25, 203–209. [Google Scholar]
- Lutz, J.A.; Furniss, T.J.; Germain, S.J.; Becker, K.M.L.; Blomdahl, E.; Jeronimo, S.A.; Cansler, C.A.; Freund, J.A.; Swanson, M.E.; Larson, A.J. Shrub consumption and immediate community change by reintroduced fire in Yosemite National Park, California, USA. Fire Ecol. 2017, 13. [Google Scholar] [CrossRef]
- Potter, C.; Dolanc, C. Thirty years of change in subalpine forest cover from Landsat image analysis in the Sierra Nevada Mountains of California. For. Sci. 2016, 62, 623–632. [Google Scholar] [CrossRef]
- Davis, B.H.; Beck, J.; van Wagtendonk, J.W. Modeling fuel succession. Fire Manag. Today 2009, 69, 18–21. [Google Scholar]
- Lutz, J.A.; van Wagtendonk, J.W.; Thode, A.E.; Miller, J.D.; Franklin, J.F. Climate, lightning ignitions, and fire severity in Yosemite National Park, California, USA. Int. J. Wildland Fire 2009, 18, 765–774. [Google Scholar] [CrossRef]
- Hurteau, M.D.; North, M. Carbon recovery rates following different wildfire risk mitigation treatments. For. Ecol. Manag. 2010, 260, 930–937. [Google Scholar] [CrossRef]
- Carlson, C.H.; Dobrowski, S.Z.; Safford, H.D. Variation in tree mortality and regeneration affect forest carbon recovery following fuel treatments and wildfire in the Lake Tahoe Basin, California, USA. Carbon Balance Manag. 2012, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Kolden, C.A.; Lutz, J.A.; Key, C.H.; Kane, J.T.; van Wagtendonk, J.W. Mapped versus actual burned area within wildfire perimeters: characterizing the unburned. For. Ecol. Manag. 2012, 286, 38–47. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Kolden, C.A. Relationships between climate and macroscale area burned in the western United States. Int. J. Wildland Fire 2013, 22, 1003–1020. [Google Scholar] [CrossRef]
- Hurteau, M.D.; Stoddard, M.T.; Fule, P.Z. The carbon costs of mitigating high-severity wildfire in southwestern ponderosa pine. Glob. Chang. Biol. 2011, 17, 1516–1521. [Google Scholar] [CrossRef]
- Fahey, T.J.; Siccama, T.G.; Driscoll, C.T.; Likens, G.E.; Campbell, J.; Johnson, C.E.; Battles, J.J.; Aber, J.D.; Cole, J.J.; Fisk, M.C.; et al. The biogeochemistry of carbon at Hubbard Brook. Biogeochemistry 2005, 75, 109–176. [Google Scholar] [CrossRef]
- Kolden, C.A.; Abatzoglou, J.T.; Lutz, J.A.; Cansler, C.A.; Kane, J.T.; van Wagtendonk, J.W.; Key, C.H. Climate contributors to forest mosaics: Ecological persistence following wildfire. Northwest Sci. 2015, 89, 219–238. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Kolden, C.A.; Lutz, J.A. Detecting unburned islands within fire perimeters using Landsat and ancillary data across the northwestern United States. Remote Sens. Environ. 2016, 186, 275–285. [Google Scholar] [CrossRef]
- Roberts, S.L.; van Wagtendonk, J.W.; Kelt, D.A.; Miles, A.K.; Lutz, J.A. Modeling the effects of fire severity and spatial complexity on small mammals in Yosemite National Park, California. Fire Ecol. 2008, 4, 83–104. [Google Scholar] [CrossRef]
- USDA Forest Service, Climate Change Land Management & Project Planning. Available online: http://www.fs.fed.us/emc/nepa/climate_change/includes/cc_nepa_guidance.pdf (accessed on 25 January 2017).
- Gower, S.T.; Vogt, K.A.; Grier, C.C. Carbon dynamics of rocky mountain Douglas-fir: Influence of water and nutrient availability. Ecol. Monogr. 1992, 62, 43–65. [Google Scholar] [CrossRef]
- Gholz, H.L.; Grier, C.C.; Campbell, A.G.; Brown, A.T. Equations for Estimating Biomass and Leaf area of Plants in the Pacific Northwest; Oregon State University School of Forestry Research Paper; Oregon State University: Corvallis, OR, USA, 1979; Volume 41, p. 39. [Google Scholar]
- Grier, C.C.; Logan, R.S. Old-growth Pseudotsuga menziesii communities of a western Oregon watershed: biomass distribution and production budgets. Ecol. Monogr. 1977, 47, 373–400. [Google Scholar] [CrossRef]
- Brown, J.K. Weight and Density of Crowns of Rocky Mountain Conifers; USFS Research Paper; United States Forest Service: Ogden, UT, USA, 1978. [Google Scholar]
- Gower, S.T.; Grier, C.G.; Vogt, D.J.; Vogt, K.A. Allometric relations of deciduous (Larix occidentalis) and evergreen conifers (Pinus contorta and Pseudotsuga menziesii) of the Cascade Mountains in Central Washington. Can. J. For. Res. 1987, 17, 630–634. [Google Scholar] [CrossRef]
- Pearson, J.A.; Fahey, T.J.; Knight, D.H. Biomass and leaf area in contrasting lodgepole pine forests. Can. J. For. Resour. 1984, 14, 259–265. [Google Scholar] [CrossRef]
- Johnston, R.S.; Bartos, D.L. Summary of Nutrient and Biomass Data from Two Aspen Sites in Western United States; USFS Research Paper 1977, INT-277:15; US Forest Service: Washington, DC, USA, 1997. [Google Scholar]
- Coltrin, W.R. Biomass Quantification of Live Trees in a Mixed Evergreen Forest Using Biomass Diameter-Based Allometric Equations. Master’s Thesis, Humboldt State University, Arcata, CA, USA, 2010. [Google Scholar]






| Consolidated Forest Vegetation Type | Number of Plots |
|---|---|
| Deciduous Oak Forest and Woodland | 88 |
| Douglas-fir Forest | 7 |
| Evergreen Oak Forest and Woodland | 110 |
| Foothill Pine Woodland | 11 |
| Foxtail Pine Forest | 58 |
| Giant Sequoia Forest | 42 |
| High Woodland | 89 |
| Jeffrey Pine Forest | 111 |
| Lodgepole Pine Forest | 190 |
| Mountain Hemlock Forest | 38 |
| Pinyon Pine Woodland | 27 |
| Ponderosa Pine Forest | 123 |
| Ponderosa Pine Woodland | 13 |
| Red Fir Forest | 116 |
| Riparian Forest | 87 |
| Riparian Shrub | 23 |
| Shrub | 258 |
| Western White Pine Forest | 27 |
| Western White Pine Woodland | 4 |
| White Fir—Sugar Pine Forest | 224 |
| Total | 1646 |
| Model Effects | AIC | ΔAIC |
|---|---|---|
| forest type | 1698.6 | 2.97 |
| forest type + burned | 1699.6 | 3.93 |
| forest type + burned + forest type × burned | 1695.7 | 0.00 |
| forest type + times burned | 1700.6 | 4.96 |
| forest type + times burned + forest type × times burned | 1698.7 | 2.98 |
| forest type + years since fire | 1699.9 | 4.26 |
| forest type + years since fire + forest type × years since fire | 1696.7 | 1.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, J.A.; Matchett, J.R.; Tarnay, L.W.; Smith, D.F.; Becker, K.M.L.; Furniss, T.J.; Brooks, M.L. Fire and the Distribution and Uncertainty of Carbon Sequestered as Aboveground Tree Biomass in Yosemite and Sequoia & Kings Canyon National Parks. Land 2017, 6, 10. https://doi.org/10.3390/land6010010
Lutz JA, Matchett JR, Tarnay LW, Smith DF, Becker KML, Furniss TJ, Brooks ML. Fire and the Distribution and Uncertainty of Carbon Sequestered as Aboveground Tree Biomass in Yosemite and Sequoia & Kings Canyon National Parks. Land. 2017; 6(1):10. https://doi.org/10.3390/land6010010
Chicago/Turabian StyleLutz, James A., John R. Matchett, Leland W. Tarnay, Douglas F. Smith, Kendall M. L. Becker, Tucker J. Furniss, and Matthew L. Brooks. 2017. "Fire and the Distribution and Uncertainty of Carbon Sequestered as Aboveground Tree Biomass in Yosemite and Sequoia & Kings Canyon National Parks" Land 6, no. 1: 10. https://doi.org/10.3390/land6010010